Cyclohexane Reaction With Bromine
What is the type of reaction between cyclohexane and bromine in dichloromethane. It does not react with bromine unless energy in the form of light or heat is applied.

Organic Chemistry Product Of Reaction Between Cyclohexene And Bromine In Methanol At 273 K Chemistry Stack Exchange
By adding bromine to a mixture of Cyclohexane and water and placing.
. The reactions of the cycloalkanes are generally just the same as the alkanes with the exception of the very small ones particularly cyclopropane. At room temperature the halogens like bromine dont react with cyclohexane. Answer 1 of 11.
The top layer is Cyclohexane and the bottom layer is water. So the correct answer is Option C. What is the equation of cyclohexane with bromine water.
The methoxy substituent present in anisole. This shows two layers with slightly different colours demonstrating that bromine is more soluble in non-polar solvents. Cyclohexene is a typical alkene and benzene and anisole are aromatic compounds.
In the case of the bonds formed a C-C single bond and 2 C-Br bonds that have no electronic transitions in the visible so when all the bromine is reacted which happens quickly the. By adding bromine to a mixture of Cyclohexane and water and placing the mixture under a bright light and shaking from time to time Hydrogen Bromide is formed. Add about 1 mL of the hexane cyclohexene and toluene to three clean test tubes The reaction of Bromine with cyclohexene in the presence of sunlight is a substitution reaction 226.
The mixture under a bright light and shaking from time to time Hydrogen Bromide is formed. Only know that cyclohexane is C6H12. The only reaction that may occur between cyclohexane and bromine is free-radical substitution.
Cumene cyanides diethyl. Reaction Of Bromine With Cyclohexane Cyclohexene And Benzene Organic Chemistry Product Of Reaction Between Cyclohexene And Bromine In Methanol At 273 K Chemistry Stack Exchange Scheme 4 A Reaction Of Cyclohexene With Electrogenerated Bromine In Download Scientific Diagram Bromine Reaction wallpaper With. Science Chemistry QA Library Consider the reaction of bromine with the pictured alkene.
The majority of examples feature only simple. Found inside Page 147Violent reaction with strong oxidizers strong reducing agents strong acids. Bromine is a dark reddish-brown fuming liquid with a pungent odor.
The reaction of bromine with sodium hydroxide forms sodium hypobromite in situ which transforms the primary amide into an intermediate isocyanate. Hydrogen bromide can be titrated with sodium hydroxide. Bromine does not react with cyclohexane without UV light with or without water.
This reaction is shown below. Intramolecular carbonyl ene reaction. Also as B r 2 is present during the reaction thus hydrogen bromide will surely be formed as a product.
Our modern society is based to a large degree on the chemicals we discuss in this chapter. When heated the -H atoms are replaced with -Br substitution. This is the answer.
Cyclohexane has no pi-unsaturation and is therefore not nucleophilic. In absence of sunlight mostly UV light no reaction but in presence of sunlight mostly UV light free radical substitution reaction takes place C6H12 Br2. Though have valid reasons further.
The electrophilic addition of bromine to cyclohexene. Cyclohexene reacts with bromine in the same way and under the same conditions as any other alkene. Therefore Bromine test differentiates Benzene and Cyclohexane by colour change.
No comments for Cyclohexane Reaction With Bromine Post a Comment. No reaction solution stay red. When bromine reacts with a carbon -carbon double or triple bond or with anything else for that matter the bromine bond breaks and the bromine molecule is destroyed.
When the bromine has all reacted and the red colour has gone the. What is the Equation of cyclohexane and bromine. Popular Which of the Following Statements Describes an Environmental Force C A car battery comes with a lifetime guarantee.
Cyclohexane and benzene both are the exceptions to the general bromine test. What is the type of reaction between cyclohexane and bromine in dichloromethane. The reaction is an example of electrophilic addition.
Ultraviolet light radiation is required for this reaction to. The reactions of the cycloalkanes are generally just the same as the alkanes with the exception of the very small ones particularly cyclopropane. What is product of cyclohexane react with bromine.
In the presence of UV light cyclopropane will undergo substitution reactions with chlorine or bromine just like a non-cyclic alkane25 Aug 2020. Denser than water and soluble in water. Youre welcome although you dont get bromocyclohexane because it doesnt react with Bromine it only dissolves in the layer.
Hence the dark brown color of the bromine water remains. Cyclohexane C6H12 Bromine Br2 --. You are not reacting bromine with cyclohexane you are DISSOLVING bromine in.
This shows two layers with slightly different colours demonstrating that bromine is more soluble in non-polar solvents. In such a case a free-radical substitution reaction occurs. See answer 1 Best Answer.
Grignard noted that alkyl halides react with magnesium metal in diethyl ether Et 2 O to form compounds that contain a metal-carbon bond. IMAGEC6H12 Br2 C6H11Br HBr. In the presence of UV light cyclopropane will undergo substitution reactions with chlorine or bromine just like a non-cyclic alkane25 Aug 2020.
Reaction Of Bromine With Cyclohexane Cyclohexene And Benzene Share.

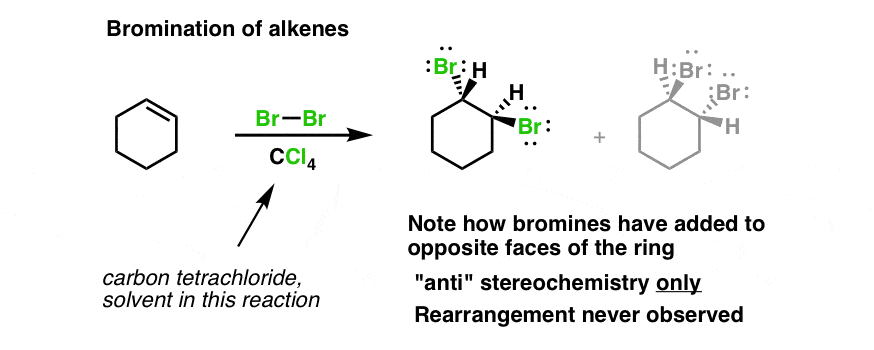
Bromination Of Alkenes Master Organic Chemistry

Perform The Calculations Necessary To Demonstrate That Bromine Is Indeed The Limiting Reagent Densities And Molecular Mass Can Be Found In Handbooks Of Chemistry For 2 Cyclohexane Br 2 2 Cyc Homework Study Com

Scheme 4 A Reaction Of Cyclohexene With Electrogenerated Bromine In Download Scientific Diagram
Reaction Of Bromine With Cyclohexane Cyclohexene And Benzene
No comments for "Cyclohexane Reaction With Bromine"
Post a Comment